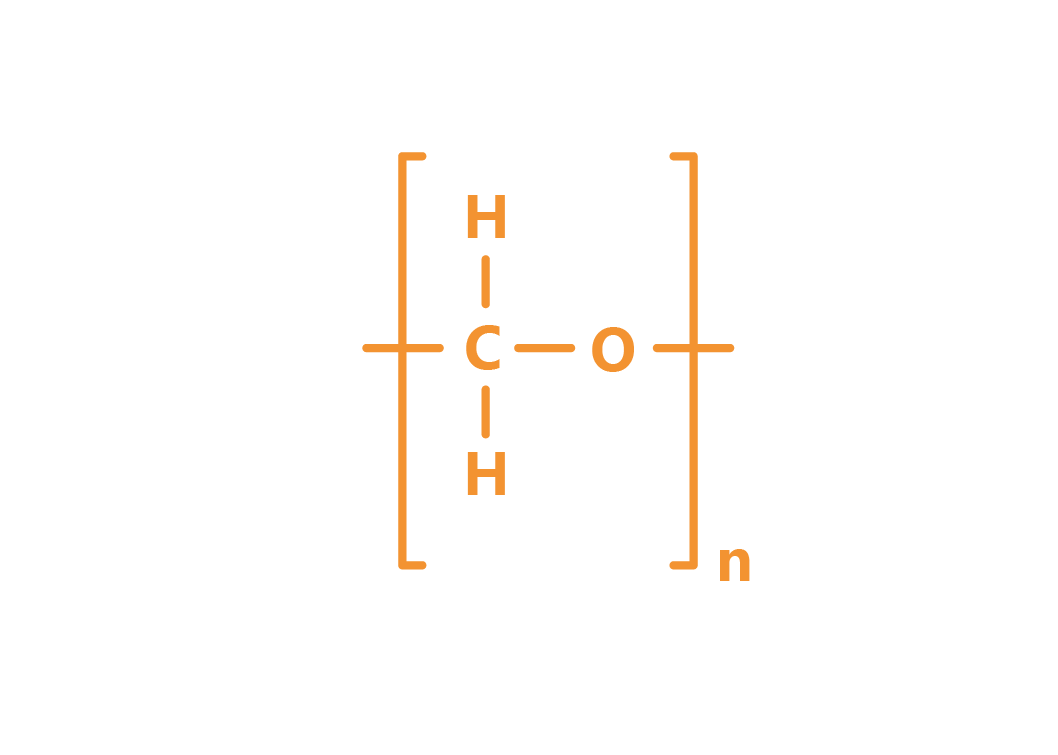
Polyoxymethylene
Polyoxymethylene (POM), also polyacetal, is produced by polymerisation of formaldehyde via intermediate steps to homoploymer or copolymer.

Abbreviation
POM
Molecular formula
(CH2O)n
CAS no. (POM-C)
24969-26-4
General description
Polyacetals (POM) are among the engineering plastics. Due to their properties such as good dimensional stability, high hardness, stiffness and strength with good toughness and chemical resistance as well as favourable sliding and abrasion behaviour, they can be used in many cases for technical articles.
POM can be modified with additives & reinforcing materials to achieve increased impact resistance and strength as well as optimised tribological properties.
Definition
Polyoxymethylene maintains its high level of strength, abrasion resistance and stiffness within a wide temperature range. In addition, the material absorbs very little water. POM has a semi-crystalline character. The crystalline content usually lies between 70 and 80 %. The melting point t is 166 °C for a copolymer and 178 °C for a homopolymer.
Production
With regard to production, a distinction is made between two basic types. Each of these results in different products: Homopolymers or copolymers.
The homopolymer is produced on the basis of formaldehyde or trioxane. In the case of formaldehyde, anionic chain polymerisation occurs. In the case of trioxane, cationic, ring-opening chain polymerisation takes place.
To produce copolymers, trioxane is reacted with ethylene oxide, dioxolane or butanediol formal. This process is called cationic, ring-opening copolymerisation.
Properties
Due to its highly crystalline nature, POM is opaque white. However, the plastic can be dyed any shade of opaque colour. Other properties of polyoxymethylene are a highly pronounced dimensional stability due to low water absorption and a high abrasion resistance.
For the plastic to burn, it is sufficient if the air surrounding it has an oxygen content of 15 %. Since air usually contains an oxygen content of around 21 %, once ignited bodies made of POM continue to burn independently.
Chemical resistance
Polyoxymethylene is resistant to alkaline solutions, petrol, diesel and oil as well as alcohols and aromatics such as benzene. The same applies to various other solvents. However, strongly halogenated solvents cause the plastic to swell. The thermoplastic is also largely resistant to acids, provided the pH value is higher than four.
Lubricants (e.g. petrol): resistant
Aromatic hydrocarbons: resistant
Esters and ketones: resistant
Alkaline solutions, ammonia, amines: resistant
Hydrogen peroxide: resistant
Water (hot): resistant
Phenols: not very resistant
Organic acids and mineral acids: not very resistant
Highly halogenated solvents: not very resistant
UV radiation: not very resistant
Processing techniques
The most common processing method is injection moulding. POM is particularly suitable for micro injection moulding. This produces small parts weighing less than two grams, whose dimensions may vary by no more than 0.3 to 0.6 %. Other common processing techniques are extrusion, blow moulding, machining, bonding and welding.
